We’re advancing research
toward a cure.
The MEF2C Family Foundation and the University of Texas Southwestern Medical Center entered a two-year Sponsored Research Agreement in 2025.
UT Southwestern will conduct innovative gene therapy research led by Dr. Steven Gray, Ph.D., in collaboration with Dr. Xin Chen, Ph.D., with the goal of advancing novel therapeutic approaches for MEF2C-related disorders.
This milestone collaboration marks a bold step toward discovering and commercializing effective gene therapies for MEF2C-related disorders, harnessing the strengths of both committed families and scientific innovation.
What’s included in the agreement?
The funding provided will allow a team of researchers and technicians at University of Texas Southwestern Medical Center to develop and explore a gene therapy construct utilizing AAV vectors with a functioning copy of the MEF2C gene.
This research is an exciting step toward uncovering potential therapies for MEF2C-related disorders like MEF2C Haploinsufficiency Syndrome.
Research Leads
Dr. Gray and Dr. Chen will dedicate a portion of their time to the project.
Staffing
A Ph.D. student, research scientist, research technician, histology technician, and lab manager will assist with the project.
Supplies
The funding will support standard lab supplies like chemicals, reagents, tubes, pipettes, bottles and more.
Animal Costs
Mice will be bred for the project, and it covers their care and veterinary costs.
What does the path to treatment look like?
Exploratory research is an essential first step toward finding a treatment for rare diseases like MEF2C Haploinsufficiency Syndrome and other MEF2C-related disorders.
If at any point in the pathway to market an exploratory treatment is deemed dangerous or ineffective, researchers and drug developers will go back to the drawing board.
Clinical Trial Process
-
Discovery & Development:
A potential new treatment is identified and studied in the lab to understand its potential.
Preclinical Research:
The treatment is tested in cells and animals to gather initial data on safety and potential effects. This phase can take several years and must show more benefit than risk to proceed.
Investigational New Drug (IND) Application:
Researchers submit preclinical data to a regulatory agency like the U.S. Food and Drug Administration (FDA). The FDA reviews the application, including the treatment's manufacturing, to ensure the trial minimizes risk to human participants.
-
Phase 0 (Exploratory): A small number of healthy volunteers receive a very low, subtherapeutic dose of the treatment to gather early data on pharmacokinetics (how the body processes the drug) and pharmacodynamics (how the drug affects the body)
-
The treatment is given to a small group of people (15-25) for the first time to evaluate safety, determine a safe dosage range, and identify potential side effects
-
A larger group of patients with the condition being studied receives the treatment to assess its effectiveness and continue monitoring safety and side effects.
-
The treatment is tested in a large, diverse population to confirm its effectiveness, compare it to standard treatments, and gather more data on side effects.
-
FDA Review: If Phase 3 results are promising, a sponsor submits a New Drug Application (NDA) to the regulatory agency, which reviews all the collected data to decide on approval.
Phase 4 (Post-Market): After approval, studies continue to monitor the treatment's safety and effectiveness in the wider, real-world population over a longer period.
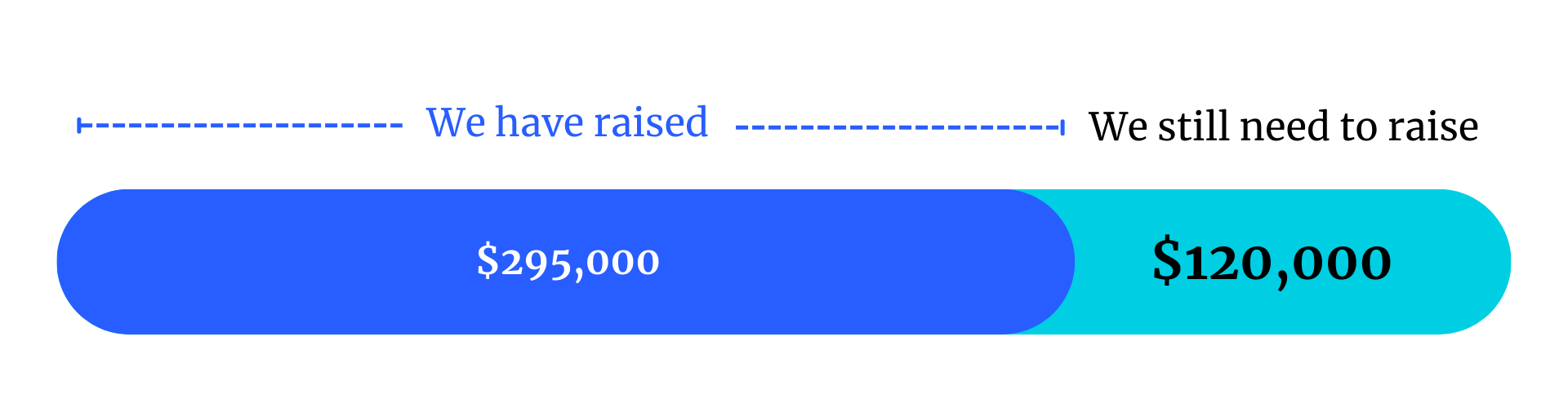
Help us raise funds
Every dollars counts — this project is made possible by community fundraising efforts.
The project costs $416,000
As of December 2025, we have raised $295,000:
Latest News
MEF2C Family Foundation and UT Southwestern Enter Pioneering Sponsored Research Agreement to Accelerate Gene Therapy for MEF2C-Related Disorders
October 15, 2025
In a major step forward for families affected by MEF2C-related disorders, the MEF2C Family Foundation and UT Southwestern have partnered on a two-year gene therapy research project. Led by Dr. Steven Gray and Dr. Xin Chen, the research team will focus on developing a new treatment that directly targets the MEF2C gene. This innovative research, set to begin in the fall of 2025, represents a major step toward creating potential therapies for children and adults with MEF2C-related disorders, which are characterized by profound developmental delays, hypotonia, intellectual disability, seizures that often begin in infancy, and a near-complete absence of speech.
The team’s research will examine the potential for an adeno-associated virus (AAV) to deliver a healthy copy of the MEF2C gene. AAV is a type of non-pathogenic virus that's become a leading vehicle for gene therapies due to its strong safety profile and ability to effectively deliver genetic material to target cells, including those in the brain.
The use of AAV has already led to major breakthroughs for other rare diseases. The FDA-approved therapies Luxturna for an inherited retinal disease and Zolgensma for spinal muscular atrophy have successfully restored vision and improved motor function, respectively, in affected patients. These successes have paved the way for further research into AAV therapies for other conditions, including hemophilia, Rett Syndrome, and various neurological disorders, offering new hope for patients with previously untreatable conditions.
“This research is a critical step towards helping our kids and loved ones who are affected by MEF2C haploinsufficiency syndrome and other MEF2C-related disorders,” said Caroline Claflin, a co-director of the MEF2C Family Foundation. “As parents, we want to do everything we can to help our kids. We’re excited to partner with Dr. Gray and his research team, who have already seen success in this area.”
Dr. Steven Gray is a distinguished leader in the field of AAV-based gene therapy, specializing in vector engineering for effective delivery to the central nervous system. He serves as professor of pediatrics, and also holds appointments in molecular biology, neurology and the Eugene McDermott Center for Human Growth and Development at UT Southwestern. Additionally, he is co-director of the Gene Therapy Program.
“Support of the MEF2C Family Foundation allows us to collaborate with other rare disease researchers to investigate pathways to potentially deliver healthy versions of this important gene, which is instrumental for cell development and differentiation in heart, brain, and skeletal muscle,” said Dr. Gray. “The enthusiasm from the foundation’s members is infectious and inspirational to these efforts.”
The project connects UTSW researchers with Dr. Cowan, chair of the Department of Neuroscience at the Medical University of South Carolina, whose research team is also tackling MEF2C-related disorders. Dr. Cowan has signed a collaborative research agreement with Dr. Gray to amplify their efforts through shared data.
This milestone collaboration marks a bold step toward discovering and commercializing effective gene therapies for MEF2C-related disorders, harnessing the strengths of committed families and scientific innovation.
About the Organizations
MEF2C Family Foundation is a nonprofit founded by families affected by MEF2C-related disorders, empowered by a mission to connect, support, and accelerate therapy development for this ultra-rare genetic condition.
UT Southwestern Medical Center is among the leading academic medical centers in the U.S. UTSW is a powerhouse in biomedical research, clinical care, and education, with notable accomplishments that include multiple Nobel laureates and leadership in regenerative science and gene therapy.